The Road to Intelligent Manufacturing: Ruijie Creates a Minimalist and Efficient Production Network Base for Pharmaceutical Enterprises

The Road to Intelligent Manufacturing: Ruijie Creates a Minimalist and Efficient Production Network Base for Pharmaceutical Enterprises
In recent years, with the acceleration of the three-medical linkage reform and the gradual implementation of normalized epidemic prevention and control measures, the living environment of pharmaceutical companies has changed, and the industry will inevitably usher in a reshaping after the reshuffle.
On the one hand, the aging of the population is increasing, and the medical needs of residents are rising. According to the forecast of the Prospective Industry Research Institute, the scale of my country's pharmaceutical market will grow at a rate of 14%-17%, and the industry scale is expected to exceed 5.3 trillion yuan by 2025; on the other hand, under the general trend of "medical insurance control costs", imitation The profit margin of pharmaceuticals has been further compressed, and the dividend has peaked. The traditional extensive model of relying on high-priced generic drugs and single drugs will give way to the innovation-driven model of "product is king".
In this context, the digital transformation of pharmaceutical companies is imperative. However, due to the weak digital foundation of the traditional pharmaceutical industry, the overall digital level of the industry is still in its infancy. The release of the "14th Five-Year Plan for the Development of the Pharmaceutical Industry" points out a clear path for pharmaceutical companies to transform—through sustainable innovation capabilities and the application of a new generation of information technology to build smart pharmaceutical factories.
How to accumulate digital capabilities and use data and tools to reduce costs and increase efficiency has become the core of digital exploration for pharmaceutical companies. However, in the process, facing the needs of production quality management and production digitalization, pharmaceutical companies still face many pain points in the digital upgrading of production networks, especially clean workshops.
The transformation of pharmaceutical companies: the pain of digital transformation of production networks
The network application of the production workshop of pharmaceutical enterprises has its distinct industry attributes.
First of all, according to the "Good Manufacturing Practice for Drugs (Revised in 2010)", the cleanliness requirements according to the four levels of A, B, C, and D run through multiple automated production scenarios such as biological culture, purification, biological preparations, and filling. .
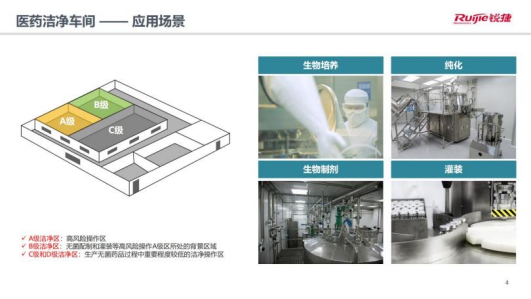
(Classification standard for clean area of pharmaceutical factory)
Furthermore, the clean workshop network needs to carry the networking requirements of various brands of equipment such as industrial computers, handheld industrial tablet mobile devices, and stationary production machine terminals.
The most important thing is that the network construction project of the clean workshop will face its own difficulties, whether it is in the technological transformation of the old workshop or the construction of the new workshop.
In the technical renovation of the old workshop:
l The network system needs to perform "surgery" such as punching, pipe threading, threading, sealing, etc. on the existing color steel plate, resulting in the production line being stopped ;
l High cost and long construction period for network system reconstruction ;
l Some production equipment has no network port, weak wireless performance, and insufficient networking capability;
l After the renovation, it is necessary to re-certify the cleanliness , and the business launch is slow.
In the new workshop scenario:
l How to install and deploy APs, especially APs in Class A clean areas, has become a prominent issue . Because the traditional panel AP protrudes from the surface of the color steel plate, it does not meet the requirements of workshop disinfection and wiping , and the indicator lights and heat dissipation holes of the ceiling type AP do not meet the workshop cleanliness certification ;
l The newly built workshops are generally large in area, and the network transmission distance is limited by 100 meters of wiring, and multiple weak current rooms need to be maintained ;
l Some production equipment has no network port, weak wireless performance, and insufficient networking capability;
l With the subsequent changes and expansion of network services, the same problems will be faced in the above-mentioned old workshop technical transformation scenario.
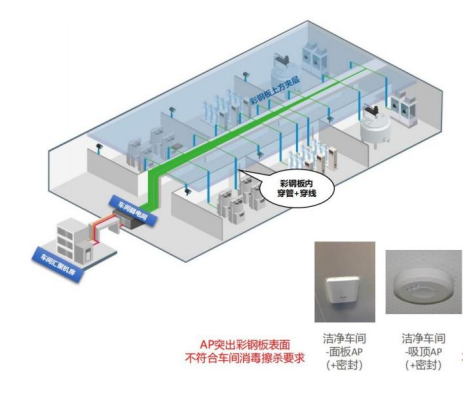
(Traditional panel APs do not meet workshop cleanliness requirements)
In response to these problems, Ruijie Networks has recently officially released a wireless solution for pharmaceutical companies' clean workshops, providing a new solution for pharmaceutical companies to smoothly advance the digital transformation of their production networks.
Ruijie's solution: a way to empower pharmaceutical companies to move forward in "medicine"
At the press conference, Lei Chunming, Director of Enterprise Industry Solutions of Ruijie Networks, described in detail the pain points that pharmaceutical companies will face in the transformation of production networks, and then pointed out: "From the whole process, we can see that if a pharmaceutical company's workshop wants to change from the original manual labor , paper-based production has become an automated, informatized, digital, and intelligent production process, which will be greatly challenged at the bottom of (digital infrastructure construction)."
The wireless solution launched by Ruijie Networks for the clean space of pharmaceutical companies directly points to the above-mentioned pain points. By integrating its advantages in the "cloud, network, and terminal" products, it has realized "simplified deployment, fast compliance" and "mobile network". Uninterrupted production" and "wireless data mining can be real-time" three values.
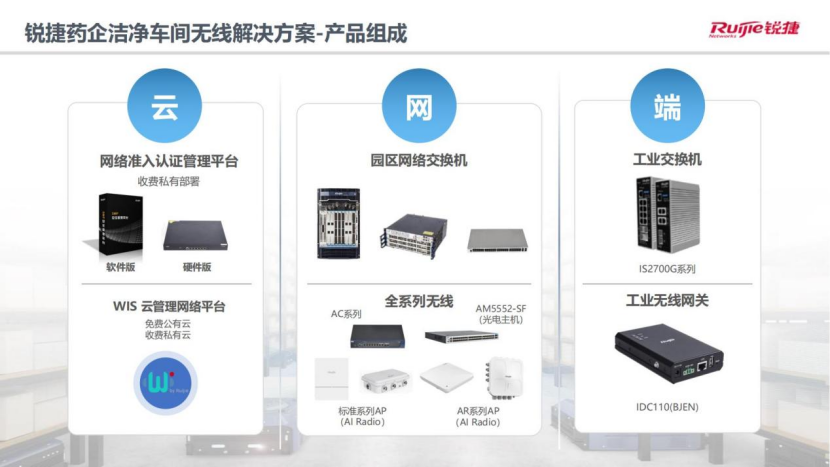
Specifically, this solution consists of three product arrays: cloud network access certification management platform and WIS cloud management platform, campus network switches and a full range of wireless products, industrial switches and industrial wireless gateways.
On this basis, an overall structure including enterprise production management system, Ruijie industrial wireless network, Ruijie industrial data collection, and production execution is constructed.
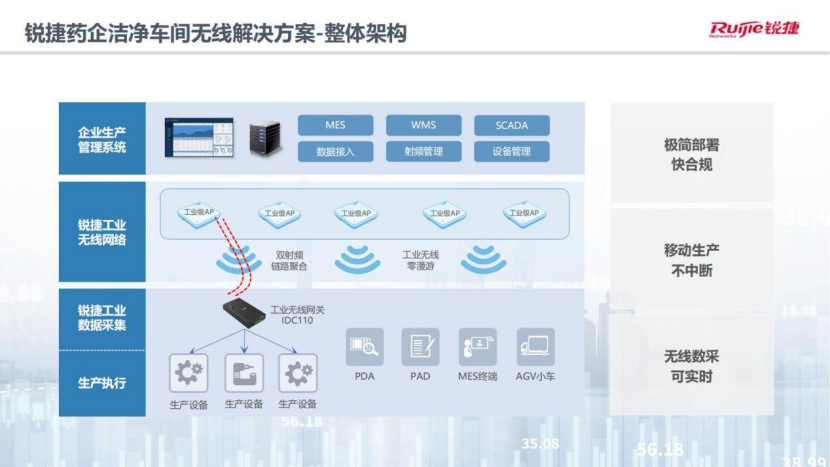
Its main value is reflected in three aspects -
"Simplified deployment and fast compliance": Ruijie Networks' "industrial-grade directional AP" plus "simplified Ethernet" is a solid guarantee for this solution to achieve "simplified deployment and fast compliance".
The penetration signal strength of industrial-grade directional AP color steel plate can reach -45dBm. By deploying one set on the interlayer above the color steel plate in each clean room, the color steel plate in the old workshop can be freed from punching/piping/threading/sealing, ensuring that the production line will not stop production. , At the same time, the construction is fast and there is no need for re-certification of cleanliness. The overall cost is low, the construction period is short, and the business launch is fast; and in the BCD-level clean area of the new workshop, ceiling AP (acrylic cover + seal) can be used to achieve rapid network deployment in the clean area. .
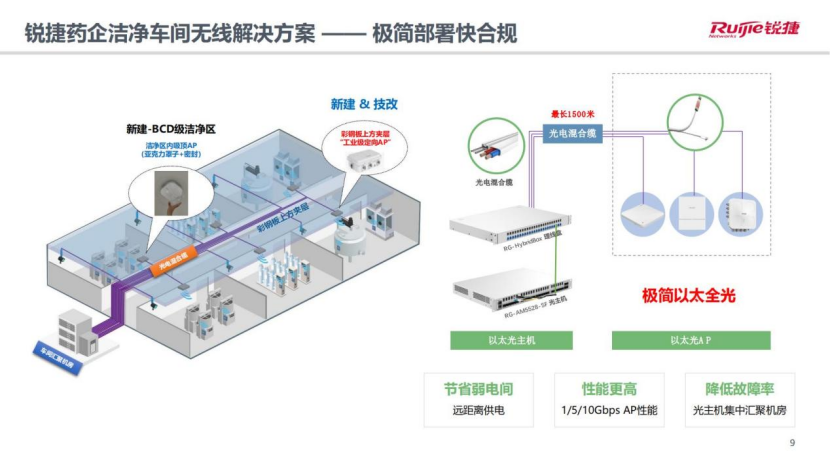
The application of Ruijie’s minimalist Ethernet technology including Ethernet host and Ethernet AP in this solution saves the weak current room (optical hybrid cable wiring length of up to 1,500 meters) for clean workshop network deployment, and brings more benefits. High AP performance (1/5/10Gbps) and lower failure rate.
"Uninterrupted mobile production" : The average delay of traditional wireless roaming is 300ms, the overall packet loss rate is 2%, and there is continuous packet loss , which often causes production personnel to hold industrial tablets and move around in several nearby clean rooms. Poor user experience of "circling in circles" or "automatic logout" in the software interface.
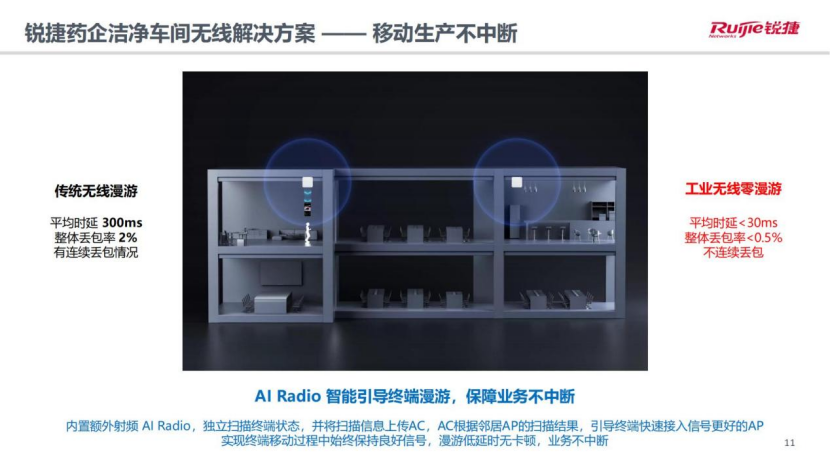
The AI Radio based on Ruijie AR series APs can actively detect terminal signal conditions, select the best AP to guide the terminal wireless roaming, the average delay is <30ms, the overall packet loss rate is <0.5%, and there is no continuous packet loss , easily achieving industrial zero roaming. Through "zero" packet loss wireless fast roaming, service insensitivity experience and MES operation efficiency, it can ensure uninterrupted mobile production of pharmaceutical companies.
"Real-time wireless data acquisition" : Using Ruijie industrial-grade wireless gateways certified by China's authoritative network equipment evaluation agency Tell Labs , the overall packet loss rate is ≤ 1/10,000, and the average delay is ≤ 10ms . In addition to the powerful industrial wireless network and industrial gateway of Ruijie Networks, the 2.4G+5G dual-RF link aggregation technology with anti-interference and high reliability is also adopted, and it supports serial/network port to wireless, which can easily achieve stable wireless data. Reliable real-time acquisition.
In addition to the three major values, this solution also has remarkable performance in terms of security assurance and operation and maintenance efficiency. Through the cloud collaboration with the WIS cloud management platform, dynamic and intelligent prediction of wireless security risks and active defense, as well as visualized wireless intelligent diagnosis and network optimization operation and maintenance are realized. According to statistics, using this solution, the wireless production network security incident and user complaint rate can be reduced by 80%, the administrator's operation and maintenance efficiency can be improved by 100%, and the fault location time can be shortened from 3 hours to 1 minute, which contributes to the efficient and safe operation of wireless networks. Provided strong support.
Epilogue
At the press conference, Li Weichuan, technical director of Beijing Chengyitong Control Engineering Technology Co., Ltd., mentioned that in 2020, the State Food and Drug Administration issued the "Good Manufacturing Practice for Drugs (Revised in 2010)", which will regulate the quality production and quality of biological products and drugs. Data tracking management puts forward clear specifications.
"There are clear requirements for the real-time capture of data in the production process of vaccine companies and the quality tracking of subsequent drugs. Therefore, various pharmaceutical companies are now upgrading intelligent manufacturing to meet the needs of their own innovation and development and ensure compliance management. Require."
Under the background of the country's strengthening of drug supervision and the structural adjustment of the pharmaceutical industry, more and more pharmaceutical companies have begun to realize the importance of digital transformation. To make a decision and act later, in order to do a good job in digital transformation, formulating a successful digital transformation strategy is the premise, and building a digital infrastructure that fits your actual situation is the cornerstone. The ultimate goal of every enterprise's digital transformation is to realize the road of business transformation based on technology and achieve data-driven business innovation and growth